PsA and COVID-19
Psoriatic Arthritis and COVID-19
Patients with rheumatic disease who are immunosuppressed or have significant comorbidities are at increased risk for serious infection. The COVID-19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, is of particular concern for these patients. Rheumatic disease and/or an immunocompromised status may place patients at higher risk for a more severe course of COVID-19, including complications, hospitalization, and death.1
Epidemiologic studies have found that patients with significant comorbidities, including cardiovascular disease, hypertension, and diabetes, are at increased risk for developing severe complications with COVID-19 infection. Current research is investigating the risk of severe COVID-19 in patients with PsA as the disease is associated with a higher incidence of comorbid cardiovascular disease, metabolic syndrome, obesity, diabetes mellitus, dyslipidemia, and inflammatory bowel disease.2
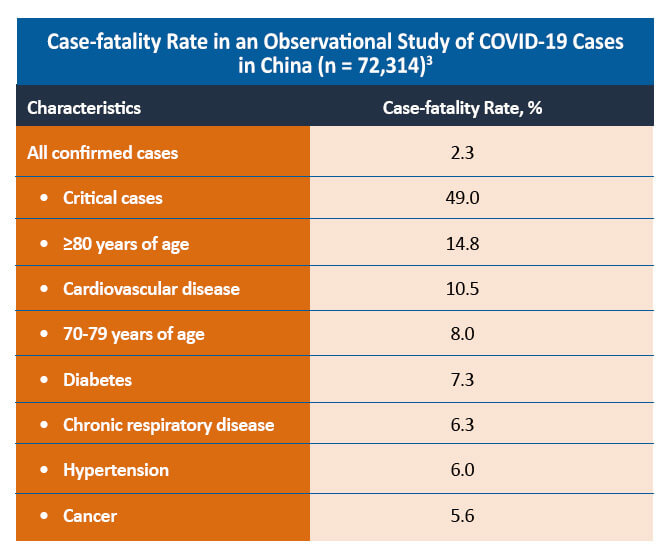
A comparative cohort study of patients with and without rheumatic disease found that both groups of patients had similar symptoms and laboratory findings. Although a similar proportion of patients with and without rheumatic disease was hospitalized in the study (44% vs 40%; P=0.50), patients with rheumatic disease were more likely to require intensive care admission and mechanical ventilation (48% vs 18%). The mortality rate was similar between the two groups (6% vs 4%; P=0.69).4
However, a small cohort study in Italy found that the presence of rheumatic disease or the degree of pharmacologic immunosuppression did not differ between patients with confirmed or suspected COVID-19 and those without. In this study, poor outcome was associated with older age and the presence of arterial hypertension and obesity.5 A large cohort study of 456 rheumatic and non-rheumatic patients found that chronic inflammatory arthritis or previous immunosuppressive therapies did not increase the risk of death, invasive ventilation, intensive care unit admission, or serious complications from COVID-19. However, older age, male sex, and previous comorbidity (obesity, diabetes, hypertension, cardiovascular or lung disease) were associated with increased risk in the rheumatic cohort.6
Treating PsA During the COVID-19 Pandemic
An additional concern for rheumatologists is the impact of PsA therapy on the risk of SARS-CoV-2 infection or the development of severe COVID-19. A case series of individuals with rheumatic disease and COVID-19 from the COVID-19 Global Rheumatology Alliance registry investigated the rate of hospitalization for COVID-19 in patients with rheumatic disease. The study found that a prednisone dose >10 mg/day was associated with a higher risk of hospitalization (OR, 2.05). However, use of conventional disease-modifying antirheumatic drugs (DMARDs) alone or in combination with biologics or Janus kinase inhibitors was not associated with an increased risk of hospitalization. No association between hospitalization and non-steroidal anti-inflammatory drugs (NSAIDs) or antimalarial use was observed. Tumor necrosis factor inhibitor (anti-TNF) use was found to significantly reduce the risk of COVID-19 hospitalization in this study (OR, 0.40).1 Another study in patients with inflammatory bowel disease and COVID-19 found that systemic corticosteroids increase the risk of severe COVID-19 (aOR, 6.9). Anti-TNF therapy was not associated with severe COVID-19 in this study (aOR, 0.9).7
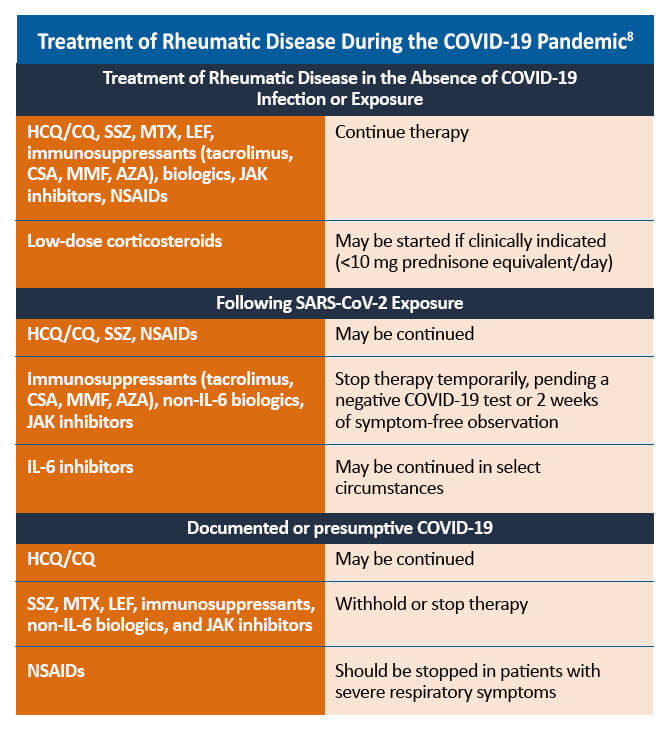
HCQ = hydroxychloroquine; CQ = chloroquine; SSZ = sulfasalazine; MTX = methotrexate; LEF = leflunomide; CSA = cyclosporine; MMF = mycophenolate mofetil; AZA = azathioprine; JAK = Janus kinase; NSAID = non-steroidal anti-inflammatory drug; IL = interleukin
Some therapies for the management of PsA may need to be withheld in patients with confirmed or suspected COVID-19. For patients with uncomplicated COVID-19 infections, such as those who experienced mild or no pneumonia treated in the ambulatory setting or by self-quarantine, therapies to manage rheumatic disease may be reinitiated within 7-14 days of symptom resolution. For patients who are asymptomatic despite a positive PCR (polymerase chain reaction) test for SARS-CoV-2, PsA therapies may be restarted 10-17 days after the positive PCR result. Decisions regarding the timing of reinitiating PsA therapies in patients who recover from more severe COVID-19-related illness should be made on a case-by-case basis.8
References
- Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalization for COVID-19 in people with rheumatic disease: Data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859-866.
- Wollina U, Fioranelli M, Goldust M, et al. Psoriatic arthritis and COVID-19 pandemic: Consequences in medical treatment? Dermatol Ther. 2020;33:e13743.
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72,314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239-1242.
- D’Silva KM, Serling-Boyd N, Wallwork R, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: A comparative cohort study from a US ‘hot spot’. Ann Rheum Dis. 2020;79:1156-1162.
- Fredi M, Cavazzana I, Moschetti L, et al. COVID-19 in patients with rheumatic disease in northern Italy: A single-centre observational and case-control study. Lancet Rheumatol. 2020;2:e549-e556.
- Pablos JL, Galindo M, Carmona L, et al. Clinical outcomes of hospitalized patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: A multicentric matched cohort study [published online ahead of print, 2020 Aug 12]. Ann Rheum Dis. 2020;218296.
- Brenner EJ, Ungaro RC, Gearry RB, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: Results from an international registry. Gastroenterol. 2020;159:481-491.e3.
- Mikuls TR, Johnson SR, Fraenkel L, et al. American College of Rheumatology Guidance for the Management of Rheumatic Disease in Adult Patients During the COVID-19 Pandemic: Version 2. Arthritis Rheumatol. 2020;72:e1-e12.










