Living with PsA
Lifestyle Changes
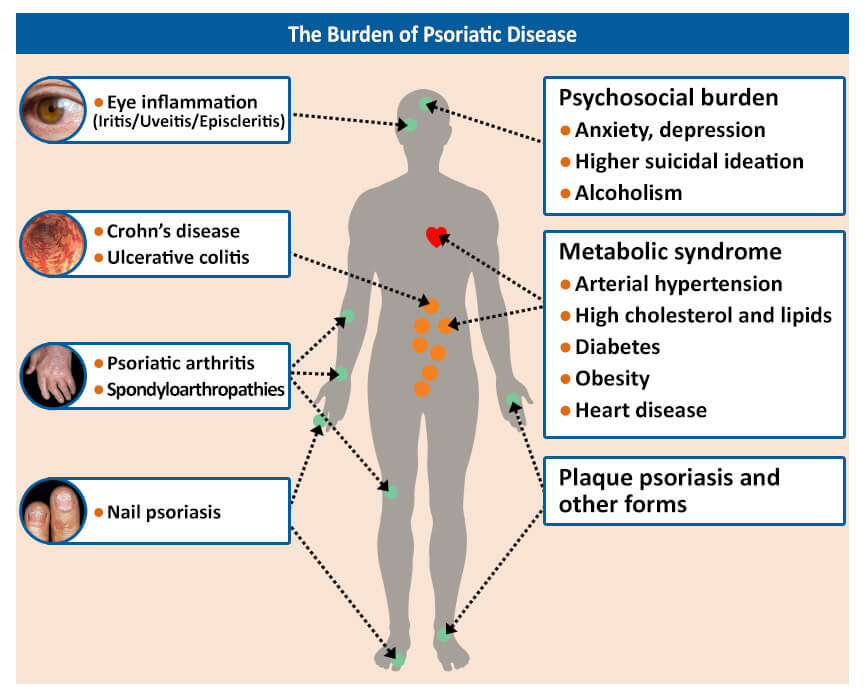
Psoriatic arthritis is a chronic condition that impacts more than just your joints and skin. It is associated with severe fatigue, depression, anxiety, and a significantly decreased quality of life. It may reduce your range of motion, making it more difficult for you to carry out daily activities. However, living with PsA is manageable if you figure out how it affects you. Over time, you will learn what triggers your flares, how to manage them, and how to avoid them.
Living a healthy life can significantly reduce the symptoms of PsA. This includes eating healthy, exercising, quitting smoking, cutting back on alcohol, losing weight, and reducing stress. Stress can both worsen and trigger symptoms of PsA, and learning to cope with stress can help to prevent flares. Physical activity can help maintain your range of motion and helps to reduce inflammation and pain. If you have joint inflammation in a weight-bearing joint in your lower extremities, swimming or working out in water can reduce the workload on your joints. Exercise will also help to lower your risk of developing other conditions that are commonly associated with PsA, such as heart disease, high cholesterol, diabetes, and obesity.
Managing Pain
The inflammation caused by PsA can lead to significant pain and swelling in the joints. Over time, this inflammation can cause long-term and permanent damage to your joints and lead to disability. Several medications are available to control pain associated with PsA.
Non-steroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen and naproxen, are available as over-the-counter medications or as prescription strength. NSAIDs decrease inflammation, joint pain, and stiffness, making it easier for you to move.
If you still experience pain with NSAIDs, your healthcare provider may prescribe a biologic therapy. Biologics target specific molecules produced by the immune system that cause inflammation. Although biologics are effective in reducing pain by targeting the underlying cause of PsA, it may take at least 3 months for biologics to reduce pain.
Weight Loss
Maintaining a healthy weight plays an important role in managing psoriatic arthritis. PsA is a chronic inflammatory condition and fat tissue produces inflammatory proteins that cause low-grade inflammation. Being overweight or obese is linked to an increased risk of developing PsA and makes it more difficult to manage. Additionally, PsA is associated with other health conditions, such as heart disease, diabetes, and metabolic syndrome. Losing weight will help prevent complications from these commonly associated conditions.
Weight loss has been linked to an improvement in PsA symptoms, including painful, swollen joints and fatigue. Weight loss does not have to be dramatic to see improvement. Research has found that overweight and obese people who lose 5% of their body weight are more likely to achieve remission. Follow the tips below to help you lose weight and improve your symptoms:
- Reduce consumption of added sugars, red meat, and full-fat dairy. These foods are higher in calories and trans fats, which can contribute to weight gain and inflammation.
- Eat more fruits, vegetables, and fish. A Mediterranean diet is recommended (2 servings of vegetables per day, 3 servings of fruit per day, and 3 servings each of legumes, nuts, and seafood per week).
- Incorporate low-impact exercise into your daily routine. Physical activity helps reduce inflammation and pain, promotes weight loss, and improves range of motion of the joints. At least 150 minutes of moderate physical activity per week is recommended.
- Lift weights two days per week to increase weight loss and improve overall fitness.
- Use a fitness tracker and set small, manageable goals.
- Keep a food journal to track calories and create accountability.
- Prioritize sleep. A lack of sleep is linked to impaired glucose metabolism and an increased risk of type 2 diabetes, metabolic changes, and inflammation. Avoid caffeine and alcohol in the evenings, use your bed only for sleeping (not for watching TV or scrolling online), and exercise regularly.
References
- https://www.psoriasis.org/living-with-psoriatic-arthritis/
- https://www.arthritis.org/home
- https://creakyjoints.org/living-with-arthritis/psoriatic-arthritis-flare-ups/
- https://www.everydayhealth.com/psoriatic-arthritis/psoriatic-arthritis-friendly-weight-loss-tips/
- Aurangabadkar SJ. Comorbidities in psoriasis. Indian J Dermatol Venereol Leprol. 2013;79(suppl 7):S10-S17.










