Diagnosis
The Importance of Early Diagnosis of Psoriatic Arthritis (PsA)
It has become increasingly clear that PsA is more common and more severe than previously thought. PsA causes joint damage and deformities that significantly impact patients’ quality of life and the disease is associated with an increased mortality risk.1 Delays in diagnosis are associated with disease progression and poorer physical functioning. One study found that patients who were diagnosed with PsA within 2 years of disease onset had less severe disease at presentation. A separate study found that a 6-month delay from symptom onset to first visit with a rheumatologist contributes to the development of peripheral joint erosion, worse long-term physical function, and poor quality of life. 2 Shorter symptom duration at diagnosis is predictive of achieving minimal disease activity (MDA).3
The degree of inflammation experienced in PsA predicts the progression of both clinical and radiological joint damage, highlighting the importance of obtaining appropriate therapy in a timely fashion to prevent joint damage progression. Additionally, joint damage is predictive of both functional limitation and mortality in patients with PsA and early diagnosis and treatment may prevent these negative outcomes.1
Raising Awareness and Screening for PsA
Despite evidence suggesting benefits with early diagnosis and therapy, a considerable diagnostic delay in patients with psoriatic arthritis remains. A study by the British Society of Rheumatology found that compared to rheumatoid arthritis, patients with PsA had significantly longer delays in initial presentation to their physician (8.9 vs 6.6 weeks), time to referral to a rheumatology clinic (5.4 vs 4.0 weeks), and time to diagnosis (28.6 vs 21.6 weeks).4
Improving screening and referral of PsA are important steps in reducing delays in diagnosis. One of the strongest predictors for the development of PsA is the presence of psoriasis. Data from the UK Clinical Practice Research Datalink found that 92.8% of patients were diagnosed with psoriasis before or within one calendar year of their PsA diagnosis. Only 7.1% of patients received a diagnosis of PsA before psoriasis.5 The incidence of PsA in patients with psoriasis is estimated to be 2.7% per year, and predictors of PsA development include severe psoriasis, psoriatic nail pitting, uveitis, and a low educational level.6
As a diagnosis of psoriasis often precedes PsA, increasing awareness of the risk of PsA development in dermatologists and primary care physicians who treat patients with psoriasis may decrease the length of time between symptom onset and referral to a specialist. Additionally, patients with psoriasis are often unaware of the risk of developing arthritis and may not report signs and symptoms of early PsA to their physician, further delaying diagnosis. Patient education and the use of screening questionnaires by dermatologists and primary care physicians may be important tools in preventing the debilitating joint damage characteristic of PsA.5
Presentation of PsA
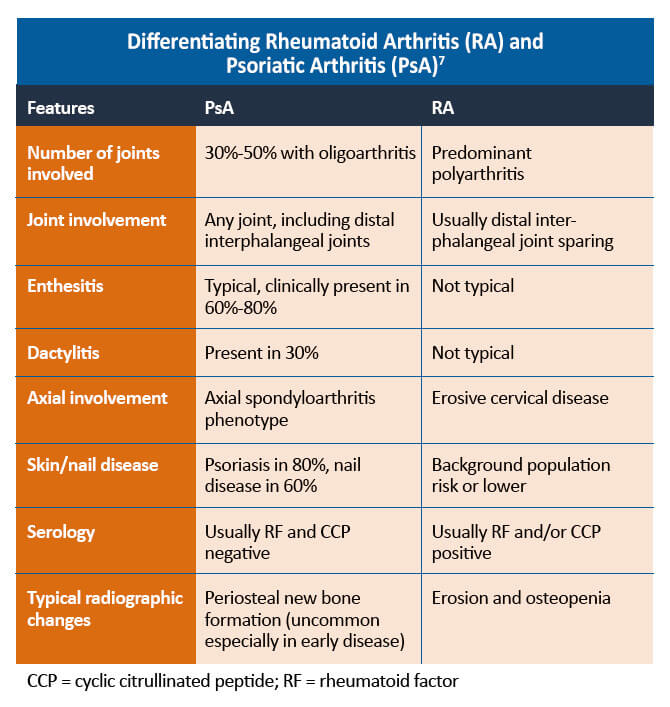
PsA is a complex disease with a heterogeneous presentation. Musculoskeletal manifestations of PsA include peripheral arthritis, spondylitis, dactylitis (inflammation of the whole digit), and enthesitis (inflammation where a tendon, ligament, or joint capsule inserts into the bone). Skin manifestations include psoriasis (most commonly psoriasis vulgaris or plaque psoriasis) and nail disease. Extraarticular manifestations include uveitis and inflammatory bowel disease. Additionally, patients with PsA often experience fatigue, limitations with physical functioning, sleep disturbances, and diminished participation in work and social activities. Several comorbidities are associated with PsA, such as obesity, depression, anxiety, and metabolic disease (diabetes, hypertension, hyperlipidemia, fatty liver disease, cardiovascular outcomes).
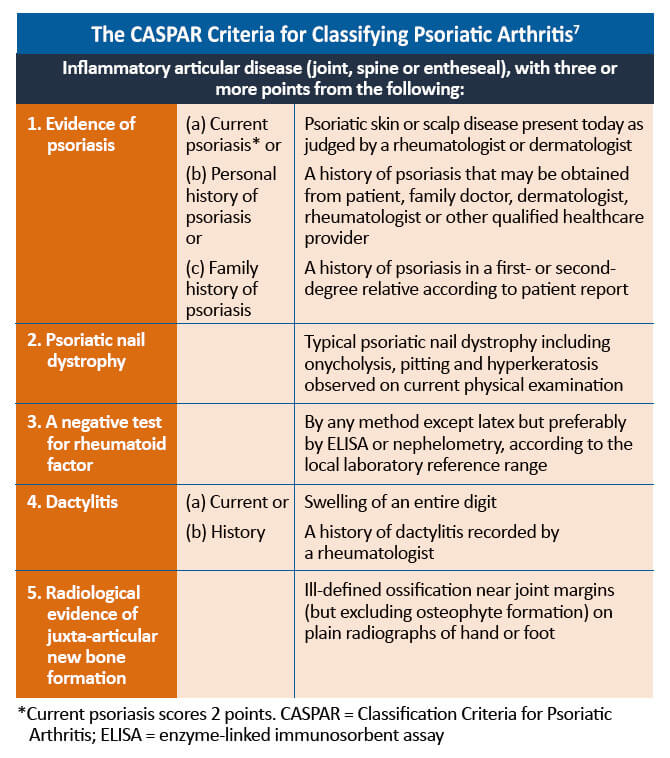
When differentiating from rheumatoid arthritis and other inflammatory arthritides, there are a number of features that can help with the diagnosis. The CASPAR (Classification Criteria for Psoriatic Arthritis) criteria define a number of features that are typical of PsA. It is important to remember that approximately 10%-15% of patients develop PsA before psoriasis. In this situation, other features, such as a family history of psoriasis, typical musculoskeletal involvement, and negative serology, can help diagnose PsA. Psoriasis may also be found in hidden areas, such as the scalp, nails, flexural areas, and natal cleft.7
References
- Gladman DD, Thavaneswaran A, Chandran V, et al. Do patients with psoriatic arthritis who present early fare better than those presenting later in the disease? Ann Rheum Dis. 2011;70:2152-2154.
- Haroon M, Gallagher P, FitzGerald O. Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis. 2015;74:1045-1050.
- Theander E, Husmark T, Alenius GM, et al. Early psoriatic arthritis: Short symptom duration, male gender and preserved physical functioning at presentation predict favourable outcome at 5-year follow-up. Results from the Swedish Early Psoriatic Arthritis Register (SwePsA). Ann Rheum Dis. 2014;73:407-413.
- Holland R, Davis A, Green A, et al. FRI0514 Psoriatic arthritis is associated with diagnostic delay and worse outcome at three months when compared to rheumatoid arthritis: Results from the UK national audit for inflammatory arthritis. Ann Rheum Dis. 2017;76(suppl 2):685.
- Van den Bosch F, Coates L. Clinical management of psoriatic arthritis. Lancet. 2018;391:2285-2294.
- Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: A prospective cohort study. Arthritis Rheumatol. 2016;68:915-923.
- Coates LC, Helliwell PS. Psoriatic arthritis: State of the art review. Clin Med (Lond). 2017;17:65-70.










